chlorine electron arrangement|electron arrangement for magnesium : Pilipinas Fluorine will gain one electron and have a charge of \(1-\). The electron . 321 uses, 25 templates - Allow me to introduce you to our popular CapCut video template - end of a film.This template has been used by over 321 people and offers 25 unique styles for users to choose from.
PH0 · oxygen electron configuration
PH1 · nitrogen electron configuration
PH2 · electronic configuration of carbon
PH3 · electron configuration of silicon
PH4 · electron configuration of cr
PH5 · electron configuration for chlorine
PH6 · electron arrangement for magnesium
PH7 · aluminium electron configuration
PH8 · Iba pa
Girls Do Porn E275 - 19 Years Old Amateur Fucked In Hotel Room [Blonde, Teen, 48m] eporner.com Open. Share Add a Comment. Sort by: Best. Open comment sort options . If you can bring yourself to go to motherless.com they have a .
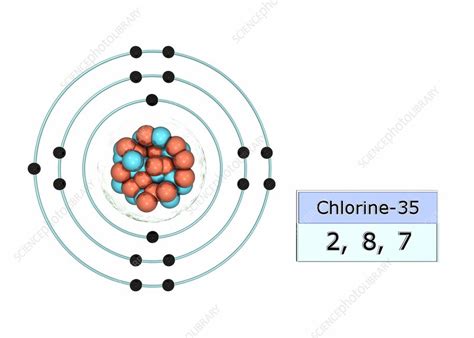
chlorine electron arrangement*******The electron arrangement of chlorine is (2, 8, 7). The electron arrangement also provides information about the number of valence electrons. The valence electrons are the electrons in the highest energy level and the ones involved in ion and bond formation.Learning Outcomes. Define mole. Determine the ratio of elements in a .
Fluorine will gain one electron and have a charge of \(1-\). The electron .Exercise \(\PageIndex{2}\): Counting Valence Electrons in Chlorine Atoms. .
A neutral chlorine atom has 17 electrons. Two electrons can go into the 1s .
In order to write the Chlorine electron configuration we first need to know the number of electrons for the Cl atom (there are 17 electrons). When we write the configuration we'll . Mar 23, 2023 Learn how to write the full and abbreviated ground state electron configuration of chlorine, a nonmetal with atomic number 17. See the table of sublevels and orbitals, and the step-by-step .chlorine electron arrangementExercise \(\PageIndex{2}\): Counting Valence Electrons in Chlorine Atoms. From the electron configuration of neutral chlorine atoms (Exercise \(\PageIndex{1}\)), how many valence electrons and how .
chlorine electron arrangement electron arrangement for magnesiumExercise \(\PageIndex{2}\): Counting Valence Electrons in Chlorine Atoms. From the electron configuration of neutral chlorine atoms (Exercise \(\PageIndex{1}\)), how many valence electrons and how . A neutral chlorine atom has 17 electrons. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. That leaves 7 .The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the . Chlorine has high electronegativity and higher electron affinity, so it reacts with almost all the elements except with lighter noble gases. Chlorine is a non explosive .

For writing the Chlorine Electron Configuration you first need to check the number of electrons for the Chlorine (Cl) atom (there are 17 electrons in chlorine atom). When you write the configuration . Chlorine (Cl) is a chemical element with the atomic number 17 and the symbol Cl. It is a non-metal in group 17 of the periodic table, also known as the halogens. Chlorine is a highly reactive element and .
What is the arrangement of electrons in chlorine 35? Two electrons in the first level, 8 in the second, 7 in the third. The electron configuration notation is: 1s22s22p63s23p5, or [Ne]3s23p5 . The electron arrangement of chlorine is (2, 8, 7). The electron arrangement also provides information about the number of valence electrons. The valence electrons are the electrons in the highest energy level and the ones involved in ion and bond formation. Knowing the number of valence electrons will allow us to .Chlorine belongs to group 17 which is known as halogens. Electronic configuration of Group 17. The elements of group 17 have seven electrons in their outermost shell. So, the valence shell electronic configuration of group 17 is n s 2 n p 5. Electronic configuration of Chlorine: The electronic configuration of fluorine is 1 s 2 2 s 2 2 p 6 3 s .Chlorine: Chlorine is an element having an atomic number 17 and an atomic symbol Cl. It belongs to the Halogen family i.e. in p-block elements. Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Electronic configuration of Chlorine:
electron arrangement for magnesiumThe electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating . The electron configuration of sodium is 1s22s22p63s1 1 s 2 2 s 2 2 p 6 3 s 1 (Table 2.7.1 2.7. 1 ). The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the element neon (Z = 10) ( Z = 10). This provides the basis for a .
Carbon, which is located in Group 4A, has 4 valence electrons. Since chlorine is found in Group 7A of the periodic table, it contains 7 valence electrons. A chemically-correct electron dot structure for each of these elements is shown below. Based on the structures shown above, carbon has 4 unpaired electrons, and chlorine .
Good team of support . The support agent is friendly and he made it easy for me when i wanted my account to be official. Because there was once a time that i have registered a ciuole of accounts just because i may have forgotten that i already registered using my other email address ( I have a couple of email addresses via Google and yahoo) So guys, if .
chlorine electron arrangement|electron arrangement for magnesium